
Clinical Trials
Innovation Programme
Empowering Pharma & Biotech Leaders to Deliver Faster, More Effective Clinical Trials

Empowering Pharma & Biotech Leaders to Deliver Faster, More Effective Clinical Trials
Clinical trials face challenges in complex protocols, competitive patient recruitment, decentralized study models, evolving FDA regulations, and multi-site data management all while keeping trials efficient and patient-focused.
Over two days, the conference will provide practical solutions to US-specific clinical trial challenges, including complex study designs, patient recruitment and retention, decentralized and hybrid models and managing multi-site data. Attendees will gain real-world case studies, and tools to run smarter, faster, and more patient-centric trials while overcoming operational and regulatory hurdles.

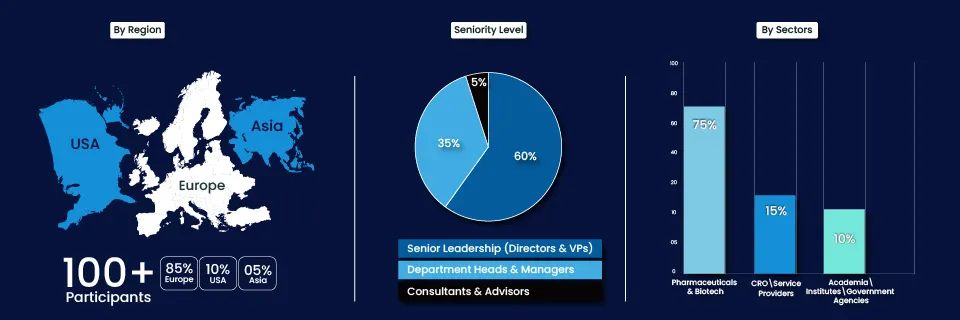
Meet the Global Community of Health Innovators
Clinical Research
Clinical Development
Clinical Operations
Site Management
Patient Advocacy
Patient Centric
Clinical Science
Regulatory Affairs
Data Management and AI
Medical Affairs
RBQM




















































